Research
1. Proteoglycans in Cartilage and Osteoarthritis
Osteoarthritis (OA) is a debilitating disease that affects more than a quarter of the population, especially the elderly. The hallmark OA is the irreversible breakdown of aggrecan in articular cartilage. Currently, there is no effective OA treatment or cartilage repair strategies.
We aim to understand how the small proteoglycan, decorin, regulates the integrity of cartilage matrix during development, degeneration and regeneration. This will be achieved by integrating our unique atomic force microscopy (AFM) nanomechanical tools, targeted decorin genetic modification in mice, chondrocyte culture and tissue engineering bioreactors. Outcomes will enable us to develop decorin-based gene therapy and biomaterials for cartilage regeneration and OA intervention.
Funding Source: NIH, NSF.
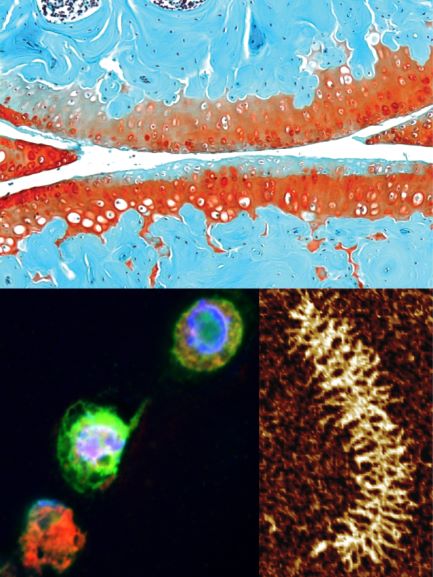
Tissue: osteoarthritic murine cartilage lacking decorin; cell: newly deposited proteoglycan; molecule: aggrecan.
2. Fibrocartilage Mechanobiology
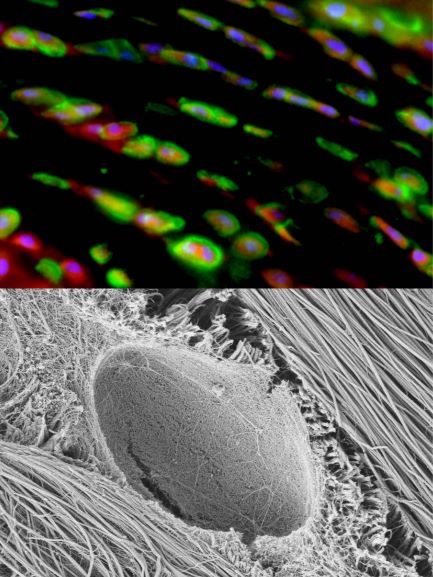
Fibrocartilage (meniscus) PCM immunofluorescence and nanostructure.
Injury and degeneration of fibrocartilaginous tissues (intervertebral disc, meniscus) have significant detrimental impacts on the quality of life. Because fibrocartilage sustains both tension and compression, its highly heterogeneous, complex meniscus ECM is one major challenge that limits the regeneration and repair.
We aim to understand how the micromechanical environment regulates the formation of this highly complex, heterogeneous structure. We will combine AFM nanomechanical tests, ECM-cell strain imaging, and intracellular calcium signaling to assess the development of native fibrocartilage.
One novel focus is the molecular activities in the pericellular matrix (PCM), the immediate cell microniche. We aim to pinpoint the activities of the PCM regulatory molecule, type V collagen, in cell-ECM mechanotransduction.
Funding Source: NIH, NSF.
3. Novel Biomaterials for Tissue Regeneration
The PCM modulates chondrocyte-matrix cross-talk, and thus mechanically regulated chondrocyte activities. It is also where initial molecular assembly of extracellular matrix takes place. One distinctive feature of the PCM is the highly concentrated, preferential distribution of proteoglycans (aggrecan, perlecan etc.). Degeneration of proteoglycans in the PCM is one of the first events in OA, which disrupts chondrocyte mechanosensing and leads to irreversible cartilage breakdown.
We aim to establish novel biomaterials based on both native and biomimetic proteoglycans to modulate hondrocyte mechanotransduction, extracellular proteoglycan assembly and thus, to promote mechanically-regulated cartilage regeneration. This project is in close collaboration with Dr. Michele Marcolongo's Biomaterials Lab (Drexel, DMSE).
Funding source: NSF.
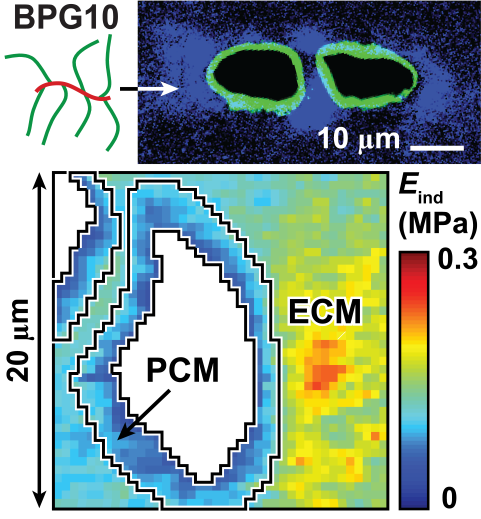
Upper: Biomimetic proteoglycan (BPG10) localized near cartilage PCM;
Lower: Nanomechanical modulus map of cartilage infiltrated with BPG10.
4. Temporomandibular Joint Disease and Regeneration
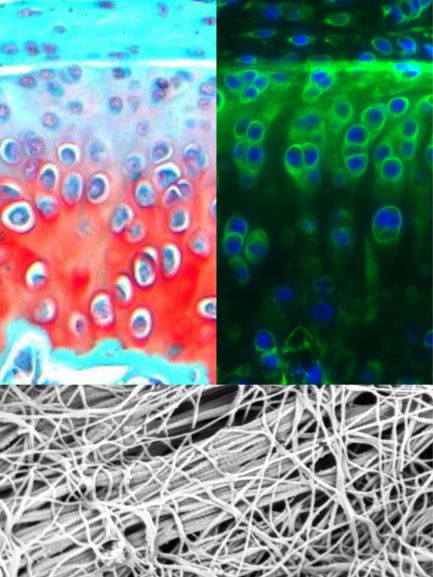
Upper: Saf-O/Fast Green histology and Collagen V IF of murine TMJ;
Lower: Collagen fibril nanostructure of murine TMJ disc surface.
Temporomandibular joint (TMJ) condylar cartilage has a unique hybrid structure: a fibrous superficial layer covering a hyaline secondary layer. TMJ disorder (TMD) is a debilitating disease that afflicts 5-12% of the US population, while the understanding of its disease pathology and success in repair are very limited.
We aim to understand how type V collagen directs the formation of this unique fibrous-hyaline hybrid tissue via multidisciplinary approaches in combining the development of conditional collagen V knockout mice, AFM nanomechanics, cell mechanotransduction, laser microdissection and TMJ injury animal models. The long term goal is to develop new strategies for repair and regeneration of TMJ condylar cartilage.
Funding Source: NIH.